Estimated Reading Time: 15 minutes

Introduction
AstraZeneca is a multinational pharmaceutical and biotechnology company, which offers a range of products targeting diseases in various fields. The company has created many blockbuster drugs and played a key role in the development of the Oxford–AstraZeneca COVID-19 vaccine. This report aims to analyse AstraZeneca’s key strategies using strategic tools such as the Ansoff Matrix and the Strategy Clock. Subsequently, it will evaluate these strategies through analytical frameworks, including the VRIN model and the PESTLE analysis.
1. AstraZeneca’s Main Strategies
1.1. Corporate-Level Strategies
1.1.1. Mission Statement
Mission statement functions as a standard corporate reporting tool (Williams, 2008) for more than 40 years. It defines and conveys the main purpose of an organisation to all its stakeholders (Kemp and Dwyer, 2003). AstraZeneca defines themselves as ‘a global, science-led, patient-focused pharmaceutical company,’ their mission is ‘to transforming the future of healthcare by unlocking the power of what science can do for people, society and the planet (AstraZeneca, 2024)’. With this prospect, they dedicate to developing new drugs for various diseases.
1.1.2. Product/market Development
The Ansoff Matrix (1957) is a strategic planning tool utilized for making deliberate decisions regarding firm growth through product and market expansion strategies (Hussain et al., 2014). The growth strategies include market penetration, market development, product development, and diversification (Hall and Lobina, 2007). In most practical scenarios, a business would concurrently implement several of these strategies (Ansoff, 1957). Judging from AstraZeneca’s current development directions, they mainly focus on product development and market development, as their activities align with both the development of products (drugs) for existing markets and the existing drugs for new markets. They have already performed quite well in market penetration strategy in the past few years. According to Pharmaceutical Executive (2024), it holds the 8th place among 50 largest global pharmacopeial biological companies based on market capitalization and prescription sales (Figure 1).
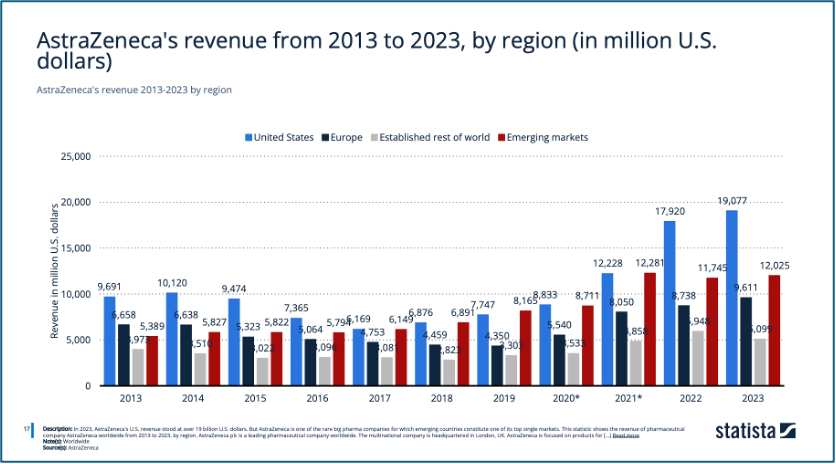
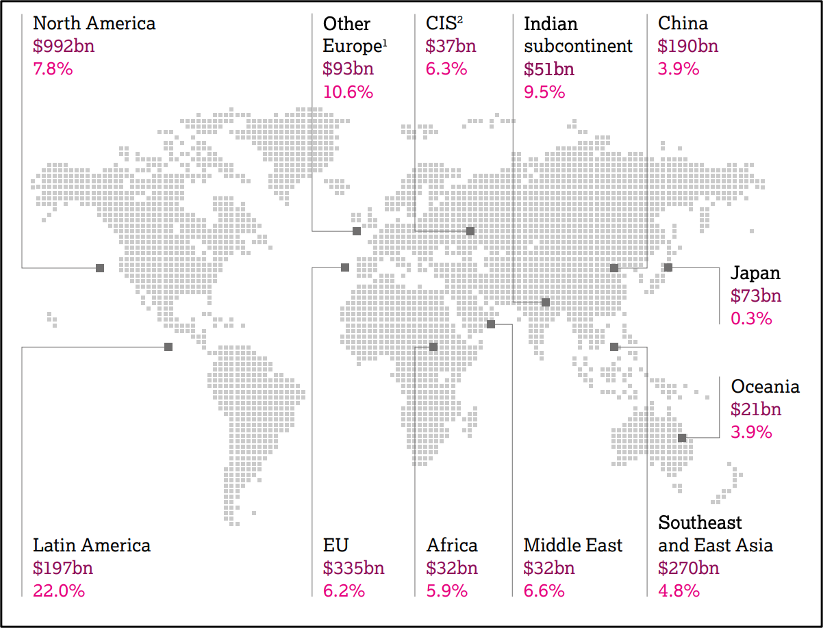
AstraZeneca is one of the few big pharma companies where emerging markets are among its top individual markets (Mikulic, 2024). They collaborate with BMS in the diabetes sector to become leaders in the non-insulin market (Team TBH, 2024), while also investing in emerging markets like China, which presents significant growth potential. Development in other emerging markets, such as India, Southeast Asia, and East Asia, also led to steady revenue growth in these regions (Figure 2). Moreover, AstraZeneca announced in July 2024 that they are set to invest ₹250 crore to expand its Global Innovation and Technology Centre in India, focusing on transforming healthcare for millions of people in India (AstraZeneca, 2024).
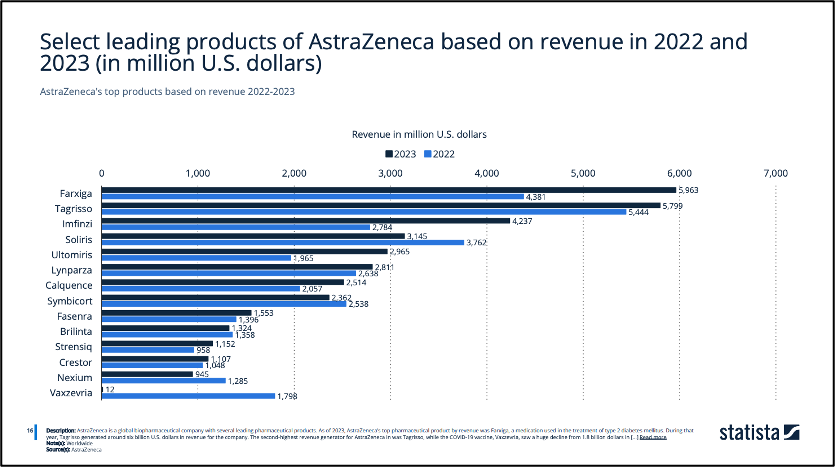
To achieve the strategy objectives, AstraZeneca strategically focusing on its key therapeutic areas and enhancing its late-stage pipeline (TheBigMarketing.com, 2024). By directing their efforts toward developing innovative medicines that meet urgent medical needs, their advanced research and development capabilities have resulted in a robust product portfolio (Statista, 2024). This contributes significantly to the success of their numerous blockbuster drugs, including Farxiga, Tagrisso and Imfinzi that brought them billions of revenues (Figure 3). The announcement of launching up to 20 new drugs, with a target to boost revenues to $80 billion by 2030 (O’Boyle, 2024), indicates AstraZeneca’s continuing focus on product development through innovation.
1.1.3. Diversification
Despite the emphasis on the two strategies mentioned earlier, diversification remains a crucial aspect of their growth strategy. Their recent actions suggest they may place even greater focus on this strategy moving forward. One of the past examples is their Oxford–AstraZeneca COVID-19 vaccine during pandemic. The development, production, and distribution of the vaccine was a significant accomplishment, establishing more than 25 supply networks across 15 countries, delivering two billion doses, and saving an estimated six million lives within the first year. (AstraZeneca, 2024).
According to Ansoff (1957), companies diversify to address technological obsolescence, manage risk, make use of excess production capacity, reinvest earnings, and attract top management. This is usually accomplished by activities such as strategic alliances, mergers and acquisitions (Cartwright and Cooper, 2012). Judging from AstraZeneca’s activities, their strategic approach appears to be horizontal integration within the framework of related diversification. This strategy involves entering new business areas that align with the same level of the value chain as the company’s existing operations (Ansoff, 1957). AstraZeneca’s acquisitions and alliances lead an expansion into new therapeutic areas closely related to its core competencies.
AstraZeneca bought a biopharmaceutical company Alexion for $39 billion in 2021. The completion of the acquisition signifies AstraZeneca’s venture into the field of rare disease treatments. In 2022, AstraZeneca stated that they will pay 660% premium for the acquisition of a gene therapy firm LogicBio, which AstraZeneca said will accelerate growth of its Alexion unit in the field of genomic medicine (Reuters, 2022). In June 2024, AstraZeneca announced the successful completion of the acquisition of Fusion Pharmaceuticals Inc., a clinical-stage biopharmaceutical company developing next-generation radioconjugates. As for alliances, AstraZeneca also actively launching partnered medicines, such as breast cancer therapy Enhertu with Daiichi Sankyo and asthma medicine Tezspire with Amgen.

1.2. Business Level Strategies
1.2.1. Strategic Business Units
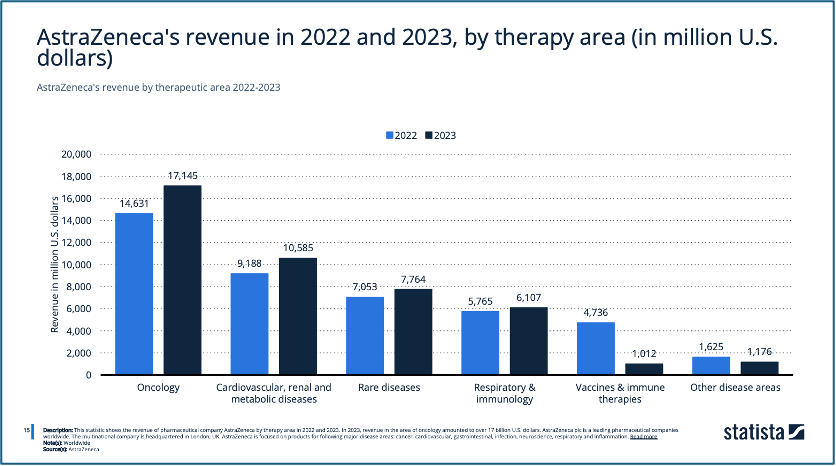
A business-level strategy is a plan developed by a company to successfully compete in a particular market or industry. It focuses on how the company will gain a competitive advantage over its rivals and achieve success in its chosen market segment (Beard and Dess, 1981). Strategic business units are tailored based on factors like the selected product, customer group, and available resources (Zimmermann, 2024). Judging from the revenue generated rankings, AstraZeneca’s business units can be divided based on their three main therapy areas – oncology, cardiovascular, renal and metabolic diseases (CVRM), and rare diseases (Figure 4).
1.2.2. Porter’s Generic Strategy Options & Bowman’s Strategy Clock
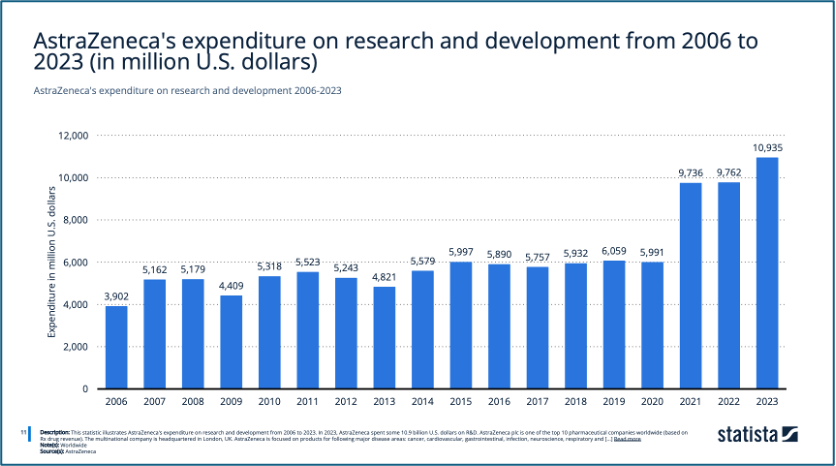
AstraZeneca’s strategic approach in these therapy areas emphasizes differentiation and focus rather than cost leadership in Porter’s Generic Strategy Options (1980). The reasons might be due to the nature of the pharmaceutical industry, where significant R&D costs (Figure 5), stringent regulatory standards, and the high expense of clinical trials make a low-cost strategy challenging to implement. Instead, AstraZeneca focuses on differentiation and innovation, developing high-quality, innovative drugs that address complex and life-threatening conditions. This allows the company to justify premium pricing, especially in specialized, high-value markets like oncology and rare diseases, where efficacy and safety are prioritized over cost. Additionally, the cost leadership strategy is more typical of generic drug manufacturers, who focus on low-cost alternatives (Porter, 1997), whereas AstraZeneca competes in a different space where innovation and uniqueness are keys. These SBUs correspondence to their differentiation and focused differentiation positions of Bowman’s Strategy Clock (1995) as well, which further refines these strategies, with a particular emphasis on the combination of price and perceived value (Faulkner and Bowman, 1995).
1.2.2.1. Differentiation
In oncology, AstraZeneca has adopted a differentiation strategy by investing heavily in research and development to create innovative and advanced therapies, such as targeted therapies and immunotherapies. These treatments are designed to offer superior efficacy and personalized medicine options, setting them apart from competitors. For example, drugs like Tagrisso and Lynparza have become leading treatments in their respective cancer types due to their effectiveness and AstraZeneca’s strong branding efforts (Adam, 2021).
AstraZeneca employs a differentiation strategy in CVRM through an integrated care approach, offering a portfolio that addresses interconnected conditions. The company’s innovative drugs, such as Farxiga and Brilinta, further reinforce this strategy by providing unique mechanisms of action that offer broad indications (Ali et al, 2023), making them stand out in the market (Wallentin et al, 2009). Since becoming CEO over a decade ago, Pascal Soriot has restructured AstraZeneca’s pipeline of new drugs, leading to the development of blockbuster treatments like the lung cancer drug Tagrisso, the leukemia drug Calquence, and the diabetes medication Farxiga. (Mathews and Fick, 2024). AstraZeneca’s focus strategy in CVRM targets specific patient groups with high unmet needs, particularly in areas where effective treatment options are limited (Kansteiner, 2023).
1.2.2.2. Focus/Focused Differentiation
In rare diseases, AstraZeneca applies a focus/focused differentiation strategy by specializing in niche markets with high unmet medical needs. Since 2011, AstraZeneca has undertaken a major revamp of its R&D strategy to enhance productivity, which had been below industry standards between 2005 and 2010. A central aspect of this new approach is focusing decision-making on five key factors: targeting the right molecule, delivering it to the right tissue, ensuring safety, selecting the right patients, and maximizing commercial potential (Morgan et al, 2018). The CEO of AstraZeneca Rare Disease (Alexion), Marc Dunoyer, stated: “Chronic hypoparathyroid patients face a significant need for an alternative to current supportive therapies, which do not address the underlying hormone deficiency. As leaders in rare disease, Alexion is uniquely positioned to drive the late-stage development and global commercialisation of eneboparatide, which has the potential to lessen the often-debilitating impact of low parathyroid hormone and avoid the risks of high-dose calcium supplementation (AstraZeneca, 2024).” The development of highly specialized, high-value treatments that are unique due to the personalized approach required for these conditions. Through its subsidiary Alexion, AstraZeneca targets smaller patient populations and capitalizes on the benefits of orphan drug status, which includes extended market exclusivity. This allows the company to dominate specific segments and charge premium prices for its treatments (BCC Publishing, 2024).
2. Evaluation of Strategy Suitability
2.1. Evaluation of Corporate Level Strategy
2.1.1. Environment Analysis
This report will be using the PESTLE framework for the environment of suitability evaluation. This framework evolves political, economic, sociological, technological, legal and environmental factors (Aguilar, 1967). However, only those factors that directly impact the main strategies will be addressed in this report.
2.1.1.1. Political
Rising geopolitical tensions and complexity could undermine the economic environment, leading to broader impacts on businesses.
2.1.1.2. Economics
Global pharmaceutical sales in 2023, average revenue grew 10.0% in Established Markets and 8.0% in Emerging Markets. This growth is expected to continue in the next few years (Figure 6), giving advantage to AstraZeneca’s market development strategy. However, the market growth in China is projected to slow, with a compound annual growth rate of 3.9%, largely due to the ongoing slowdown of the major hospital sector (AstraZeneca, 2023).
2.1.1.3. Sociological
Low- and middle-income countries (LMICs) are experiencing significant population shifts, with projections indicating that by 2050, two-thirds of the global population aged 60 and over will reside in these regions. LMICs are also disproportionately affected by noncommunicable diseases (NCDs), which cause 41 million deaths annually. In 2019, NCDs accounted for seven of the ten leading causes of death, representing 74% of global mortality. Specifically, cardiovascular diseases are responsible for 17.9 million deaths each year, followed by cancers with 9.3 million, chronic respiratory diseases with 4.1 million, and diabetes with 2.0 million (WHO,2022). AstraZeneca’s strong innovative development can be addressing these future trends and gain great benefits.
2.1.1.4. Technological
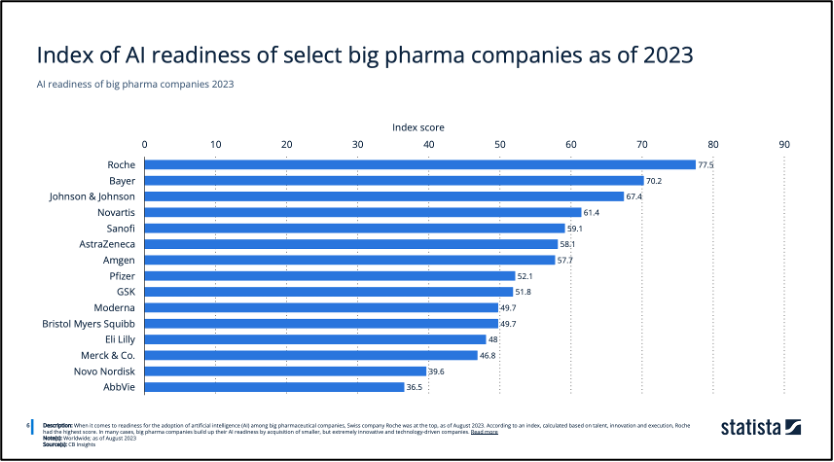
Artificial Intelligence (AI) has recently begun expanding its applications across various sectors, with the pharmaceutical industry emerging as a key beneficiary. There is a significant impact of AI in multiple areas within the pharmaceutical sector, including drug discovery and development, drug repurposing, enhancing pharmaceutical productivity, and improving clinical trials (Paul, et al, 2021). The Index of AI Readiness measures how prepared and advanced these companies are in adopting and integrating artificial intelligence into their operations, research, and development processes. Notably, AstraZeneca ranked 6th in among select big pharma companies as of 2023 (Figure 7), showing their advanced development in technology.
2.1.1.5. Legal
Enhertu, a groundbreaking drug developed through the collaboration between AstraZeneca and Daiichi Sankyo, showed a clinically significant improvement in progression-free survival for patients with HER2-ultralow expression (AstraZeneca, 2024). However, after commercial discussions involving NICE (National Institute for Health and Care Excellence), the NHS, and the drug manufacturer (Daiichi Sankyo and AstraZeneca), NICE decided not to recommend the drug due to its unreasonable and unaffordable pricing (Johnston, 2024). They stated that treatments need to be priced fairly for both the NHS and taxpayers. In 2022, NICE revised its methodology for evaluating medicines to prioritize those addressing severe diseases. Despite the changes, Enhertu was still assessed as too costly given the price established by its manufacturers. NICE has indicated that it would be open to re-engaging in commercial discussions if the companies present a fair and reasonable pricing proposal (DHSC Media Team, 2024).
2.2. Evaluation of Business Level Strategy
2.2.1. Environment Analysis
2.2.1.1. Economic
Economic instability and currency fluctuations in different regions can impact revenue and profitability. AstraZeneca’s diversified global market presence helps mitigate risks associated with economic volatility in individual countries. Moreover, AstraZenecahas enough financial resources to compete against its rivalries and conquer the leading market with its innovation and creativity and high investment in R&D (Singh et al. 2021). Adequate financial resources and continuous investment in R&D help the organisation to maintain its long-term success.
2.2.1.2. Sociological
By 2030, one in six people globally will be aged 60 years or older. The population of those aged 80 and above is expected to triple by 2050, reaching 426 million (World Health Organization, 2022). This indicated the growing demand for innovative medicines to meet urgent medical needs, reinforcing AstraZeneca’s emphasis on product development.
2.2.1.3. Technological
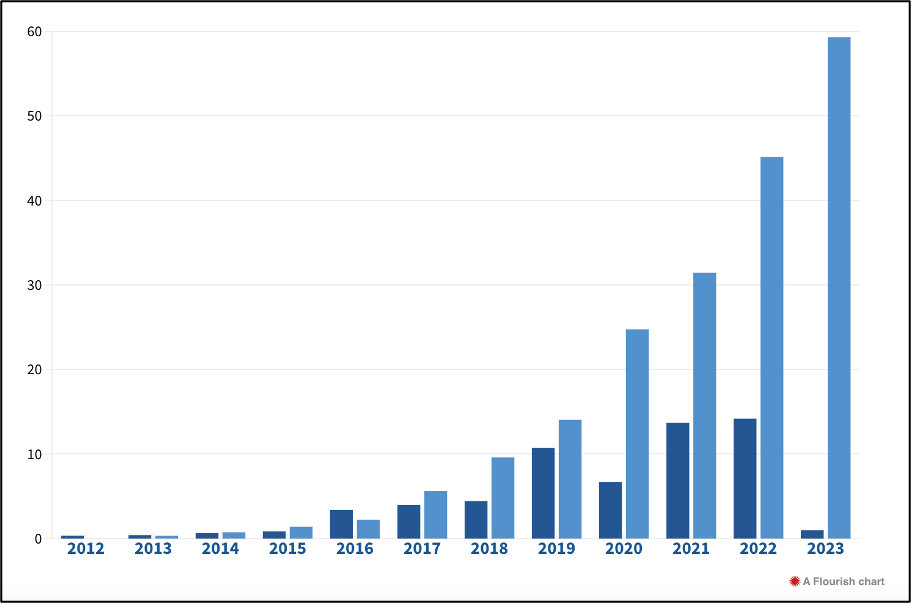
Investment in AI-enabled drug discovery is estimated to have grown 27-fold in the past nine years (Figure 8), exceeding $60 billion (Deep Pharma Intelligence, 2023). AstraZeneca’s R&D IT leverages technology to enhance the speed and quality of drug discovery and delivery. The team focuses on optimizing scientists’ workflows through advanced IT solutions, while also driving transformation in R&D by developing and implementing new digital and data science platforms aimed at increasing success rates and shortening timelines. Their expertise spans automation, analytics-ready data, scalable engineering, high-quality digital applications, seamless integration, and technology innovation (AstraZeneca, 2022).
2.2.1.4. Legal
Compliance with local and international regulations is vital for market expansion. AstraZeneca must navigate varying legal requirements across different countries, impacting their ability to successfully implement market development strategies. Pharmaceutical companies often use strategic patenting to drive up costs. Although this practice is currently legal, beyond causing high drug prices, strategic patenting impedes innovation from both original developers and generic producers (Gurgula, 2020). In May 2024, the US District Court for the District of Delaware has directed AstraZeneca to pay $107.5 million in damages to Pfizer for violating patents associated with the lung cancer medication Tagrisso. These patents, which Pfizer has licensed to Puma Biotechnology, are crucial for the production of the breast cancer drug Nerlynx.
2.3. Capabilities Analysis
This author will be using Barney’s VRIN framework (1990) to assess the organisation’s capabilities for sustainable competitive advantage.
2.3.1. Value ✔️
AstraZeneca often employs value-based pricing, even for heart drug Brilinta, where the price reflects the therapeutic benefit a drug offers to patients. This approach aligns with their focus on improving patient outcomes and healthcare system efficiency. AstraZeneca has also made an agreement with UPMC based on a “two-sided financial risk” model. Under this arrangement, UPMC’s payment for Brilinta will vary depending on the drug’s value to its health plan. If patient outcomes are better than expected, UPMC will receive a smaller discount, but if the value falls short of expectations, the discount will increase (Helfand, 2019).
2.3.2. Rarity ✔️
AstraZeneca has a portfolio of leading drugs that have achieved significant market success and revenue. Drugs like Tagrisso, Imfinzi, and Farxiga are market leaders in their respective therapeutic areas. The unique combination of efficacy, safety, and strong market presence makes these drugs a rare asset. Moreover, their strong R&D program and dedication to rare diseases provide them with a significant competitive advantage in terms of rarity.
2.3.3. Robustness ✔️
AstraZeneca maintains a robust R&D presence in over 60 countries worldwide, with key strategic R&D centers located at The DISC in Cambridge, Gaithersburg, Maryland in the greater Washington, D.C. area, and Gothenburg, Sweden. Additionally, the company recently announced the establishment of a new center in Kendall Square, Boston, MA, and through the acquisition of Alexion, added a Rare Disease R&D Centre of Excellence in New Haven, Connecticut, US (AstraZeneca, 2024). AstraZeneca’s integrated R&D teams and streamlined decision-making processes leverage unique scientific capabilities, contributing to one of the most productive pipelines in the industry (Humphreys, 2023).
2.3.4. Non-substitutability −
AstraZeneca’s patents on its products are at risk of being contested by third parties. This could lead to the introduction of generic alternatives, which might result in the revocation, circumvention, or invalidation of these patents. Furthermore, a number of AstraZeneca’s products are already encountering generic competition in various markets. AstraZeneca may face the risk of losing market share due to patent expirations and market overlap with competitors.
2.3.5. Dynamic Capability ✔️
AstraZeneca’s research and development efforts are organized into distinct yet interconnected areas. The Oncology R&D division is dedicated to advancing cancer therapies. BioPharmaceuticals R&D focuses on Cardiovascular, Renal, and Metabolism (CVRM), Respiratory and Immunology, Vaccines and Immune Therapies, and selectively explores Neuroscience. The Rare Disease R&D unit is committed to addressing rare diseases across various therapeutic areas. These divisions operate both independently and in collaboration, supported by shared functions that specialize in essential scientific disciplines (AstraZeneca, 2024). These include medicinal chemistry, biometrics, patient safety, data science, artificial intelligence (AI), clinical innovation, and device technology (Hoots, 2024). This coordinated approach enhances AstraZeneca’s ability to develop and execute a highly dynamic and effective strategic capability.
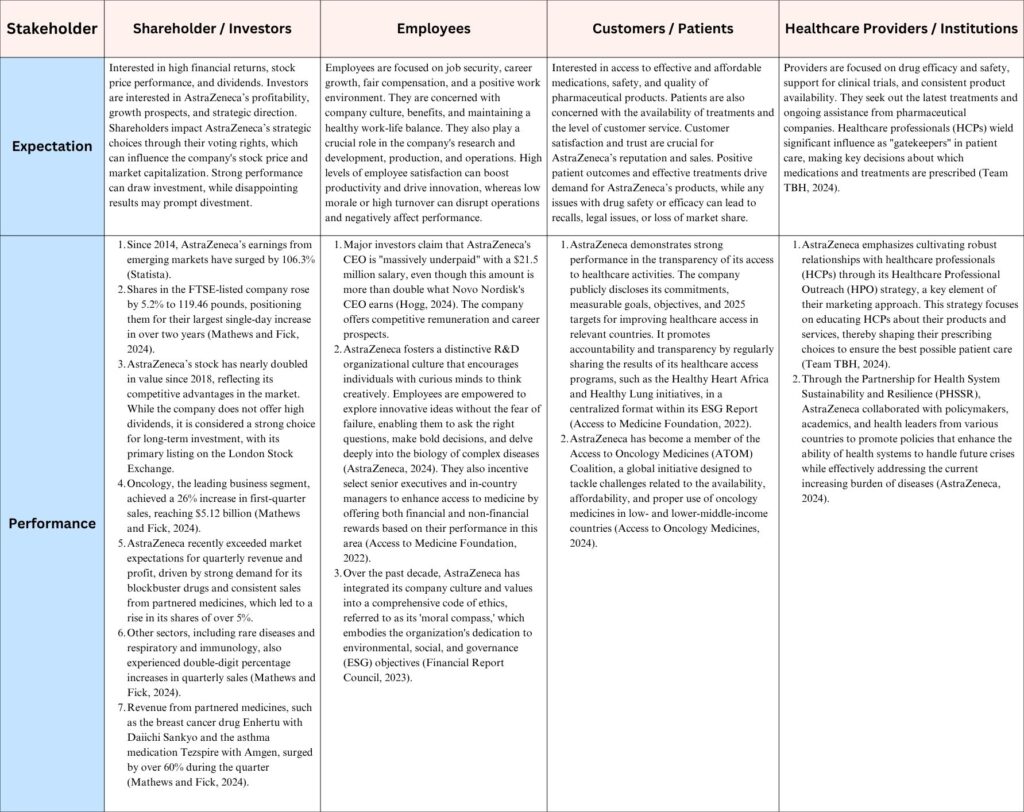
2.4. Stakeholder Expectations
The Chart below shows the four main stakeholder groups of AstraZeneca, their expectations and strategies related performances of the organisation.
3. Conclusion
Through comprehensive and critical analysis and evaluation, it is evident that AstraZeneca’s main strategies are well executed and largely align with the overall suitability evaluation. However, there are several potential threats, including regulations concerning patents, negotiations over drug pricing, and a highly competitive market. AstraZeneca must continue to advance and maintain a dynamic capability to address future challenges.
4. References
Ansoff, H. I. (1957). Strategies for diversification. Harvard Business Review, 35(5), p. 114.
Aguilar, F. J. (1967). Scanning the business environment, New York: Macmillan.
Adam, B. (2021). ‘AstraZeneca’s Tagrisso to lead niche lung cancer market with $7B sales potential’, FiercePharma, 12 October. Available at: https://www.fiercepharma.com/marketing/astrazenecas-tagrisso-lead-niche-lung-cancer-market-7b-sales-potential-report
AstraZeneca. (2022). ‘AstraZeneca R&D: Turning science into medicine’, AstraZeneca. Available at: https://www.astrazeneca.com/content/dam/az/r-and-d/pdf/turning-science-into-medicine.pdf
Access to Medicine Foundation. (2022). ‘AstraZeneca plc’, Access to Medicine Foundation, Available at: https://accesstomedicinefoundation.org/company/astrazeneca-plc#
AstraZeneca. (2023). ‘AstraZeneca Annual Report and Form 20-F Information 2023’, AstraZeneca. Available at: https://www.astrazeneca.com/investor-relations/annual-reports/annual-report-2023.html
Ali,A. E., et al. (2023). Effect of Dapagliflozin in Patients with Heart Failure: A Systematic Review and Meta-Analysis. Global heart, 18(1), p. 45. https://doi.org/10.5334/gh.1258
AstraZeneca. (2024). ‘AstraZeneca’s revenue in 2022 and 2023, by therapy area’, Statista. Statista Inc.. Available at: https://www.statista.com/statistics/293056/astrazeneca-revenue-by-therapeutic-area/
Access to Oncology Medicines. (2024). ‘Access to Oncology Medicines Coalition’, Access to Oncology Medicines. Available at: https://www.uicc.org/atom/coalition-partners
AstraZeneca. (2024). ‘AstraZeneca plans to invest Rs 250 crore to grow its Global Innovation and Technology Centre in India, bringing some 1,300 highly skilled roles by 2025’, AstraZeneca. Available at: https://www.astrazeneca.in/media/press-releases/2024/astrazeneca-plans-to-invest-to-grow-its-gitc-in-india.html
AstraZeneca. (2024). ‘Coronavirus (COVID-19) information & research hub’, AstraZeneca. Available at: https://www.astrazeneca.com/our-therapy-areas/vaccines-and-immune-therapies/covid-19.html
AstraZeneca. (2024). ‘Our Company’, AstraZeneca. Available at: https://www.astrazeneca.com/our-company.html
AstraZeneca. (2024). ‘AstraZeneca to acquire Amolyt Pharma,
expanding late-stage rare disease pipeline’, AstraZeneca Media. Available at: https://www.astrazeneca.com/media-centre/press-releases/2024/astrazeneca-to-acquire-amolyt.html
Beard, D. W., & Dess, G. G. (1981). Corporate-level strategy, business-level strategy, and firm performance. Academy of management Journal, 24(4), pp. 663-688. https://journals.aom.org/doi/abs/10.5465/amr.1988.4306865
BCC Publishing. (2024). ‘Global Markets for Orphan Drugs’, BCC Research. Available at: https://www.bccresearch.com/market-research/pharmaceuticals/orphan-drugs-market-report.html
Cartwright, S., and Cooper, C. L. (2012). Managing mergers acquisitions and strategic alliances, Routled.
Deep Pharma Intelligence. (2023). ‘Artificial Intelligence for Drug Discovery’, Deep Pharma Intelligence. Available at: https://www.deep-pharma.tech/ai-in-dd-q1-2023-subscribe
DHSC Media Team. (2024). ‘Enhertu: Everything You Need to Know’, GOV.UK. Available at: https://healthmedia.blog.gov.uk/2024/07/30/enhertu-everything-you-need-to-know/
Faulkner, D. and Bowman, C. (1995). The Essence of Competitive Strategy, New York; London: Prentice Hall.
Financial Report Council. (2023). ‘AstraZeneca – aligning and integrating ethical culture with ESG to drive competitiveness’, Financial Report Council. Available at: https://www.frc.org.uk/library/standards-codes-policy/corporate-governance/corporate-culture/astrazeneca/
Gurgula O. (2020). Strategic Patenting by Pharmaceutical Companies – Should Competition Law Intervene?. IIC International Review of Industrial Property and Copyright Law, 51(9), pp. 1062–1085. https://doi.org/10.1007/s40319-020-00985-0
Hall, D., & Lobina, E. (2007). Profitability and the poor: Corporate strategies, innovation and sustainability. Geoforum, 38(5), pp. 772-785. https://doi.org/10.1016/j.geoforum.2006.08.012
Hussain, S., et al. (2014). Interactive effects of Ansoff growth strategies and market environment on firm’s growth. British Journal of Business and Management Research, 1(2), p. 69. https://www.gbjournals.org/wp-content/uploads/interactive-effects-of-ansoff-growth-strategies-and-market-environment-on-firm%E2%80%99s-growth.pdf
Helfand, C. (2019). ‘AstraZeneca’s latest value-based pact serves up Brilinta at generic-level copays’, Fierce Pharma, 28 Jan. Available at: https://www.fiercepharma.com/pharma/astrazeneca-s-latest-value-based-pricing-pact-serves-up-brilinta-at-generic-like-prices
Humphreys, A. (2023). ‘Pipelines Report 2023: innovation and diversification’, Pharma Live. Available at: https://www.pharmalive.com/pipelines-report-2023-innovation-and-diversification/?__cf_chl_tk=5Et8Q3ySvsFYmFTpdEgKYJDbpMXUq8zpH3wVEIU._DQ-1723529036-0.0.1.1-3860
Hoots, C. (2024). ‘AstraZeneca: Where data and AI impact lives’, Health Awareness. Available at: https://www.healthawareness.co.uk/patient-care/astrazeneca-where-data-and-ai-impact-lives/
Hogg, R. (2024). ‘Major investors say AstraZeneca boss is ‘massively underpaid’ on $21.5 million salary, despite earning more than double Novo Nordisk’s CEO’, Fortune, 10 April. Available at: https://fortune.com/europe/2024/04/10/astrazeneca-boss-pascal-soriot-pay/
Johnston, I. (2024). ‘AstraZeneca calls on UK to change rules on innovative drug pricing’, Financial Times, 25 July. Available at: https://www.ft.com/content/466a4d1d-e559-4695-ba77-92364c6b3447
Kemp, S., and Dwyer, L. (2003). Mission statements of international airlines: a content analysis. Tourism Management, 24(6), pp. 635-653. https://doi.org/10.1016/S0261-5177(03)00049-9.
Kansteiner, F. (2023). ‘With FDA nod, AstraZeneca’s Farxiga gains ground in heart failure race with Jardiance’. Fierce Pharma, 5 September. Available at: https://www.fiercepharma.com/pharma/fda-nod-astrazenecas-farxiga-gains-ground-heart-failure-race-jardiance
Morgan, P., et al. (2018). Impact of a five-dimensional framework on R&D productivity at AstraZeneca. Nature Reviews Drug Discovery, 17(3), pp. 167-181. https://doi.org/10.1038/nrd.2017.244
Mathews, E., and Fick, M. (2024). ‘AstraZeneca leaps after smashing first-quarter forecasts’, Reuters, 25 April. Available at: https://www.reuters.com/business/healthcare-pharmaceuticals/astrazeneca-beats-first-quarter-revenue-profit-estimates-2024-04-25/
Mikulic, M. (2024). ‘AstraZeneca’s revenue from 2013 to 2023, by region’, Statista. Statista Inc.. Available at: https://www.statista.com/statistics/266550/revenue-of-astrazeneca-by-region-since-2006/
O’Boyle, D. (2024). ‘New era of growth: AstraZeneca sets out bold plans for $80bn revenues by 2030’, The Standard, 21 May.Available at: https://www.standard.co.uk/business/astrazeneca-sets-out-bold-plans-for-80bn-revenues-by-2030-b1159167.html
Porter, M. E. (1980). Competitive Strategy: Techniques for Analyzing Industries and Competitors, New York: Free Press.
Porter, M. E. (1997). Competitive strategy. Measuring business excellence, 1(2), pp. 12-17. https://scholar.google.co.uk/citations?view_op=view_citation&hl=zh-TW&user=g9WIbh0AAAAJ&citation_for_view=g9WIbh0AAAAJ:8d8msizDQcsC
Paul, D., et al. (2021). Artificial intelligence in drug discovery and development. Drug Discovery Today, 26(1), pp. 80–93. https://doi.org/10.1016/j.drudis.2020.10.010
Pharmaceutical Executive. (2024). ‘Leading 50 global pharmaceutical companies by prescription sales and R&D spending in 2023 (in billion U.S. dollars)’, Statista. Statista Inc.. Available at: https://www.statista.com/statistics/273029/top-10-pharmaceutical-companies-sales-and-rundd-spending-in-2010/
Statista. (2024) ‘Select leading products of AstraZeneca based on revenue in 2022 and 2023’, Statista. Statista Inc.. Available at: https://www.statista.com/statistics/267807/astrazeneca-top-products-based-on-revenue/
Statista. (2024). ‘Projected global pharmaceutical sales for 2027, by region’, Statista. Statista Inc.. Available at: https://www.statista.com/statistics/299694/world-pharmaceutical-sales-by-region-forecast/
Team TBH. (2024). ‘Marketing Strategy and Marketing Mix of AstraZeneca’, The Brand Hopper. Available at: https://thebrandhopper.com/2024/02/25/marketing-strategy-and-marketing-mix-of-astrazeneca/
TheBigMarketing.com. (2024). ‘AstraZeneca Marketing Strategy 2024: A Case Study’, TheBigMarketing.com. Available at: https://thebigmarketing.com/astrazeneca-marketing-strategy/
Williams, L. (2008). The mission statement: A corporate reporting tool with a past, present, and future. The Journal of Business Communication, 45(2), pp. 94-119. https://doi.org/10.1177/0021943607313989
Wallentin, L., et al. (2009). Ticagrelor versus clopidogrel in patients with acute coronary syndromes. The New England journal of medicine, 361(11), pp. 1045–1057. https://doi.org/10.1056/NEJMoa0904327
World Health Organization. (2022). ‘Ageing and health’, World Health Organization. Available at: https://www.who.int/news-room/fact-sheets/detail/ageing-and-health
Zimmermann, A. (2024). Topic 4 – Business Level Strategy [lecture slides], 23BSP028: Global Strategic Management, Loughborough University. Available at: https://learn23.lboro.ac.uk/course/view.php?id=17415§ion=3